Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
TB Diagnostics: Existing Platform and Future Dir
*Corresponding author:Hussein Hassan Twabi, Helse Nord Tuberculosis Initiative, Kamuzu University of Health Sciences (KUHeS), Malawi-Liverpool-Welcome Trust Clinical Research Programme, London School of Hygiene and Tropical Medicine, USA..
Received: February 05, 2022; Published: March 22, 2022
DOI: 10.34297/AJBSR.2022.15.002169
Introduction
For over 200 years, tuberculosis (TB) has been the leading infectious killer in the world [1] and has only been paralleled by COVID-19 over the past 2 years [2]. About 10 million people developed TB globally and 1.3 million died in 2020 [1]. Despite progresses made in global TB control, TB remains a major health concern, with drug resistance growing in proportion [1]. The COVID-19 pandemic also confounded efforts to end the plague of TB as outlined by the End TB strategy [1, 3]. Rapid, early diagnosis and timely management of drug-susceptible TB combined with universal drug susceptibility testing (DST) is essential in the efforts to end the TB pandemic [1]. However, most people affected by TB do not have access to essential diagnostics and expertise to diagnose TB early, and less so have access to adequate DST [4]. This article gives an overview of the current diagnostic platform for active TB in adults and briefly reviews the gaps in TB diagnostic algorithms in low resource settings
Available Diagnostics for Active TB
Low-resource settings such as Malawi have limited access to reliable electricity and internet connectivity. As such, the main diagnostics available in these settings are sputum smear microscopy, nucleic acid amplification tests, antigen detection tests, chest radiography (as a screening modality) and cultures [4].
Chest X-Rays (CXRs)
Chest radiography is a highly sensitive tool that can be used for triaging TB suspects in facility or community settings [5,6]. It has a high throughput and is relatively cheap compared to other TB tests and imaging modalities [7-9]. However, CXRs have low specificity and high inter- and intra-observer variability, making it difficult for use in health facilities. Additionally, lack of experts required to interpret CXRs reliably result in its limited use in primary care settings [7].
As such, CXRs were historically utilized towards the end of diagnostic algorithms for TB, after multiple sputum microscopy examinations were performed with inconclusive results in TB suspects [5]. The advent of digital chest radiography has reduced the limitations of conventional x-ray (including poor image or viewer quality and the cost of setup and logistics) [5]. The digital chest radiography also allows for treatment monitoring and stratification of TB cases into low versus high-risk groups for stratified treatment approaches [10].
Recent developments in the field of digital chest radiography have incorporated computer-aided diagnostics technology into it, allowing for a tremendous increase in the sensitivity and specificity of CXRs in the diagnostic pathway of tuberculosis [11,12]. The World Health Organization has not yet published a guideline for the use of computer aided CXR (CADXR) technology in the diagnostic algorithm for TB [9]. However, it has acknowledged emerging evidence of its usefulness and proposes further questions to be explored by researchers to inform guideline development [9]. CADXR thus has the potential to change the landscape of TB triage and screening.
Sputum Smear Microscopy
Sputum smear microscopy was developed over 100 years ago [13] and is the basis for TB diagnosis in low-resource primary health care settings. It is relatively fast, inexpensive, and specific for TB in high incidence areas [14]. However, it is dependent on a high bacillary load, quality of the specimen and the training and motivation of laboratory personnel,[14] resulting in a varying sensitivity, reaching as low as 20% in specific populations [14,15]. Sputum smear microscopy may be labor-intensive, have considerable patient costs (due to delayed diagnosis and repeated visits to deliver multiple samples) and inconvenience associated with the need to submit multiple sputum specimens over a period of up to three days [16].
Nucleic Acid Amplification Tests (NAAT)
The most commonly available NAAT in Malawi is the Xpert MTB/RIF. This cartridge-based PCR test offers rapid diagnosis of Mycobacterium tuberculosis as well as limited drug susceptibility testing (rifampicin resistance testing) with limited dependence on operator skill [4]. Xpert MTB/RIF has a high sensitivity and specificity for detecting TB in HIV negative individuals, but lower accuracy in HIV positive individuals. [17,18]. Xpert MTB/RIF is limited in its ability to distinguish between live and dead bacilli, meaning the assay may remain positive even after treatment completion, and, thus, should not be used to monitor response to treatment [19]. Constraints to widespread rollout include cost, need for continuous power supply, sensitivity to high temperatures, and assay throughput [4]. The novel Xpert assay, the Xpert MTB/ RIF Ultra, shows higher sensitivity at the cost of specificity in HIV positive individuals [20,21]. It is also better suited for low resource settings due to its faster turnover and better performance in high temperatures [22]. However, cost and reliance on stable power supplies are still limitations.
Antigen Detection Tests
Urine lipoarabinomannan (LAM) tests are the most widely used antigen detection tests in low resource settings. LAM is a mycobacterial cell wall glycolipid that has a profound effect on the innate immune response [23]. The STAMP trial - a pragmatic, multicenter, parallel-group, double-blind, randomized controlled trial on rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa – found that the addition of a urine-based tuberculosis screening using TB-LAM and Xpert to sputum-based screening in all HIV-positive medical inpatients significantly increased tuberculosis diagnoses and treatment across all patients cost-effectively, and significantly reduced the 56- day mortality in pre-specified high-risk subgroups [24, 25]. Several advances in the LAM test have substantially improved the diagnostic accuracy of this antigen detection test in both HIV-positive and HIVnegative populations [26,27].
Culture
Culture is the gold standard for the diagnosis of tuberculosis and permits the diagnosis of drug resistance and emerging mutations [14]. Widely used methods include solid media (e.g., Lowenstein- Jensen media) and liquid-culture media (e.g., mycobacterial growth indicator tube (MGIT)). TB culture takes a long time to produce definitive results (may take 4 to 8 weeks with an additional 4 weeks for drug sensitivity testing) and requires biosafety facilities that are expensive to build and maintain, uninterrupted power supply and highly trained laboratory technicians to perform the procedure [4,14]. Culture is hence not widely available in low resource settings in primary and secondary health care facilities, relying on tertiary centers and national reference laboratories to offer this service to them [4,14]. This presents the additional problem of sample transportation and result acquisition after the 4 to 8 weeks.
Drug Sensitivity Tests (DST)
DST is performed using either phenotypic methods or genotypic methods. Commonly known phenotypic methods are commercial culture, speciation and DST which are not widely available in low resource settings, are logistically challenging to access, and take a long time to generate results [4]. The Xpert MTB/RIF assay is a genotypic drug resistance test for rifampicin. The PCR targets an 81-bp region of the rpoB gene of M. tuberculosis where more than 95% of mutations associated with rifampin resistance occur [4]. Xpert MTB/RIF has limited application in drug sensitivity testing, but its wide availability and relatively faster throughput has led many countries to use it in their guidelines, managing any case of rifampicin resistance observed through Xpert MTB/RIF testing as multidrug resistant (MDR) tuberculosis [4]. Line probe assays (LPA) are other highly sensitive and specific genotypic tests for the detection of rifampicin resistance in culture isolates [4, 28]. The test has lower sensitivity when used directly on clinical specimens [28].
Daignostic Algorithms
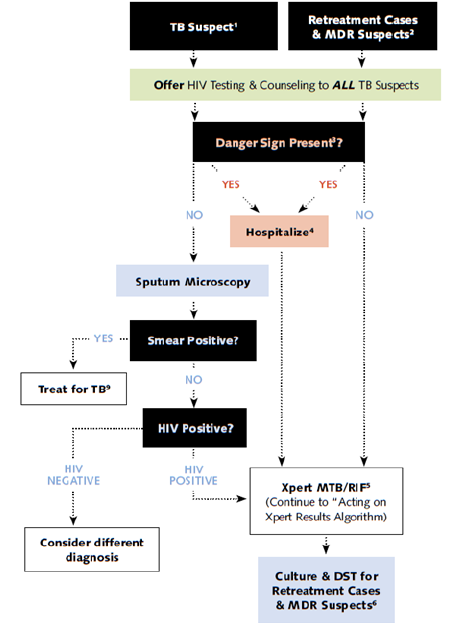
Current diagnostic pathways in many low resource countries, including Malawi, are informed by resource availability and outdated evidence. They rely heavily on symptom screening and sputum smear microscopy - [Figure 1 & 2] [29]. There is significant patient losses-to-follow-up at each stage of the care pathway [30]. Smear microscopy is a low accuracy test that relies on bacterial load and sputum sample quality, especially in HIV-positive populations [14,15]. Additionally, symptom screening is not a reliable method of screening for TB. A Lancet meta-analysis showed that a median of only 68.0% (IQR 37.0–86.4) of people living with HIV had at least one of the four symptoms [30]. Efforts are required to explore various algorithms that incorporate diagnostics such as CXR and TB-LAM at community intensified case finding levels, primary care levels and referral care levels.
Direction of TB Diagnostics
There are numerous diagnostic tests in development today,
including host-response tests, host biomarker tests, molecular
diagnostics and antigen assays inspired by the success of Xpert
MTB/RIF. The COVID-19 pandemic has also raised new problems
with regards to the diagnosis of TB, especially with the overlapping
of symptoms. The direction of priority test development and
research needs some guidance to ensure prioritization of key
revolutionary tests. Kik et al [31]. summarized priority tests as
follows:
• A point-of-care sputum-based test as a replacement for smear
microscopy.
• A point-of-care, non-sputum-based test capable of detecting
all forms of TB.
• A point-of-care triage test, which should be a simple, low-cost
test for use by first-contact health care providers as a rule-out
test.
• Rapid DST at microscopy center level
These priority areas include considerations of cost and throughput, considerations of delays in diagnosis of TB, and diagnosis of tuberculosis in children. Another proposed area for diagnostics research is the joint platform for the diagnosis of both TB and COVID-19. Additional tests that need to be prioritized are tests for latent TB infection and tests that will enable monitoring of patient treatment progress.
Conclusions
The diagnostic platform for tuberculosis is rapidly changing. These novel diagnostics are beginning to reshape the landscape of diagnosis of tuberculosis globally, including in low-resource settings. However, these diagnostics are yet to be used effectively in the diagnostic cascade. There is hence a growing need for the restructuring of diagnostic algorithms in such settings, with additional consideration for combined TB-COVID-19 screening. The effective use of diagnostics has the potential to greatly impact patient care and outcomes, thus being a key driver towards achieving the End TB Strategy goals [32,33].
References
- World Health Organization (WHO). Global Tuberculosis Report 2021. Geneva, Switzerland.
- WHO Coronavirus (COVID-19) Dashboard: WHO Coronavirus (COVID-19) Dashboard with Vaccination Data.
- Nzawa R, Burke RM, Feasey HRA, Wakumanya Sibande, Marriott Nliwasa, et al. (2021) Impact of COVID-19 on tuberculosis notifications in Blantyre Malawi: an interrupted time series analysis and qualitative study with healthcare workers.
- Madhukar Pai, Mark P Nicol, Catharina C Boehme (2016) Tuberculosis diagnostics: State of the art and future directions. Tuberc Tuber Bacillus Second (Edn) 4(5).
- Bornali Datta, Ashish Prakash, David Ford, Jaya Prasad Tripathy, Pinky Goyal, et al. (2020) Implementing upfront mobile digital chest x-ray for tuberculosis diagnosis in India-feasibility and benefits. Trans R Soc Trop Med Hyg 114(7): 499-505.
- Evangelia Skoura, Alimuddin Zumla, Jamshed Bomanji (2015) Imaging in tuberculosis. International Journal of Infectious Diseases 32: 87-93.
- Faiz Ahmad Khan, Tripti Pande, Belay Tessema, Rinn Song, Andrea Benedetti, et al. (2017) Computer-aided reading of tuberculosis chest radiography: Moving the research agenda forward to inform policy. Eur Respir J 50(1): 1700953.
- Madlen Nash, Rajagopal Kadavigere, Jasbon Andrade, Cynthia Amrutha Sukumar, Kiran Chawla, et al. (2020) Deep learning, computer-aided radiography reading for tuberculosis: a diagnostic accuracy study from a tertiary hospital in India. Sci Rep 10(1):210.
- WHO (2016) Chest Radiography in Tuberculosis. World Heal Organ 1-44.
- Marjorie Z Imperial, Payam Nahid, Patrick P J Phillips, Geraint R Davies, Katherine Fielding et al. (2018) A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med 24(11): 1708-1715.
- Khan FA, Majidulla A, Tavaziva G, Ahsana Nazish, Syed Kumail Abidi, et al. (2020) Chest x-ray analysis with deep learning-based software as a triage test for pulmonary tuberculosis: a prospective study of diagnostic accuracy for culture-confirmed disease. Lancet Digit Heal 2(11): e573-e581.
- Zhi Zhen Qin, Melissa S Sander, Bishwa Rai, Collins N Titahong, Santat Sudrungrot, et al. (2019) Using artificial intelligence to read chest radiographs for tuberculosis detection: A multi-site evaluation of the diagnostic accuracy of three deep learning systems. Sci Rep 9(1): 15000.
- (2020) World Health Organization. Global Tuberculosis Report 2020. Geneva, Switzerland.
- Seema Oommen, Nandita Banaji (2017) Laboratory diagnosis of tuberculosis: Advances in technology and drug susceptibility testing. Indian J Med Microbiol 35(3): 323-331.
- Linda M Parsons, Akos Somoskövi, Cristina Gutierrez, Evan Lee, C N Paramasivan, et al. (2011) Laboratory diagnosis of tuberculosis in resource-poor Countries: Challenges and opportunities. Clin Microbiol Rev 24(2): 314-350.
- (2009) World Health Organization, Tropical Disease Research: For research on diseases of poverty. Approaches to Improve Sputum Smear Microscopy for Tuberculosis Diagnosis: Expert Group Meeting Report.
- Karen R Steingart, Ian Schiller, David J Horne, Madhukar Pai, Catharina C Boehme, et al. (2014) Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014(1): CD009593.
- Grant Theron, Jonny Peter, Richard van Zyl-Smit, Hridesh Mishra, Elizabeth Streicher, et al. (2011) Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 184(1): 132-140.
- Sven O Friedrich, Andrea Rachow, Elmar Saathoff, Kasha Singh, Chacha D Mangu, et al. (2013) Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 1(6): 462-470.
- Susan E Dorman, Samuel G Schumacher, David Alland, Pamela Nabeta, Derek T Armstrong, et al. (2018) Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 18(1): 76-84.
- O Opota, J Mazza-Stalder, G Greub, K Jaton (2019) The rapid molecular test Xpert MTB/RIF ultra: towards improved tuberculosis diagnosis and rifampicin resistance detection. Clin Microbiol Infect 25(11): 1370-1376.
- Cepheid. Xpert® MTB/RIF Ultra, https://www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-RIF-Ultra (accessed 21 May 2021).
- Margarida Correia-Neves, Christopher Sundling, Andrea Cooper, Gunilla Källenius (2019) Lipoarabinomannan in Active and Passive Protection Against Tuberculosis. Front Immunol 10: 1968.
- Gupta Wright A, Corbett EL, van Oosterhout JJ, et al (2018) Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 392(10144): 292-301.
- Reddy KP, Gupta Wright A, Fielding KL, et al (2019) Cost-effectiveness of urine-based tuberculosis screening in hospitalised patients with HIV in Africa: a microsimulation modelling study. Lancet Glob Heal 7(2): e200-e208.
- Tobias Broger, Mark P Nicol, George B Sigal, Eduardo Gotuzzo, Alexandra J Zimmer, et al (2020) Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J Clin Invest 130(11): 5756-5764.
- Tobias Broger, Mark P Nicol, Rita Székely, Stephanie Bjerrum, Bianca Sossen, et al (2020) Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: A meta-analysis of individual in- A nd outpatient data. PLoS Med 17(5): e1003113.
- Madhukar Pai, Jessica Minion, Hojoon Sohn, Alice Zwerling, Mark D Perkins (2009) Novel and Improved Technologies for Tuberculosis Diagnosis: Progress and Challenges. Clin Chest Med 30(4): 701-716,
- (2012) Ministry of Health Malawi National Tuberculosis Programme. Malawi National Tuberculosis Control Programme Manual. 7th Editio.
- Helena R A Feasey, Elizabeth L Corbett, Marriott Nliwasa, Luke Mair, Titus H Divala, et al (2021) Tuberculosis diagnosis cascade in Blantyre, Malawi: a prospective cohort study. BMC Infect Dis 21(1): 178.
- Sandra V Kik, Claudia M Denkinger, Martina Casenghi, Caroline Vadnais, Madhukar Pai (2014) Tuberculosis diagnostics: Which target product profiles should be prioritised? Eur Respir J 44(2): 537-540.
- (2015) World Health Organization Executive Board. Global strategy and targets for tuberculosis prevention, care, and control after 2015, November 2013, pp. 1-23, 2015. Geneva, Switzerland.
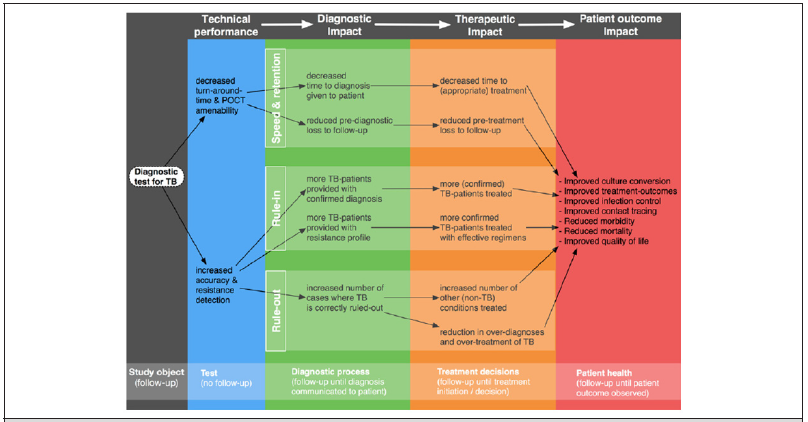
- Samuel G Schumacher, Hojoon Sohn, Zhi Zhen Qin, Genevieve Gore, J Lucian Davis, et al (2016) Impact of molecular diagnostics for tuberculosis on patient-important outcomes: A systematic review of study methodologies. PLoS One 11(3): e0151073.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.